
This is shown in the periodic table by atomic number, which increases as you move along the period. The atomic number of an element is equal to its number of protons, and is also equal to its number of electrons in a neutral atom. All elements in the same period have the same number of electron shells. As you move across a period, the number of electrons increases by one each time. For example, all the elements in group 3 have three electrons in their outer shell. The group of an element tells you how many electrons it has in its outer shell.

The period of an element tells you how many electron shells it has - for example, elements in period 2 all have two electron shells. The period and group of an element on the periodic table tell you a lot about its electron configuration. Periodic trends in electron configuration The properties we'll look at today include:įirst up - electron configuration. This might seem like a lot to learn about, but as you'll see, they show regular periodic trends when it comes to certain properties. All in all, there are seven periods and eighteen groups in the periodic table. Columns in the periodic table are known as groups. Periodic table trendsĪs we saw above, rows in the periodic table are known as periods. You can find more information on this topic in our article on the periodic table. We'll also delve into the history and structure of the periodic table. In this article, we'll take a closer look at some of the trends he observed and explain why they occur. Mendeleev was the first to notice periodicity in the table and arranged the elements accordingly. In the case of the elements, periodicity means the repetition of properties after a certain atomic number. Another word you might hear is periodicity, which refers to the repetition of properties after a certain interval.
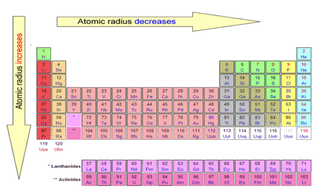
These trends are patterns that repeat themselves as you move across a period or down a group. It consists of rows, called periods, and columns, called groups, and is designed to show periodic trends. The periodic table is a systematic arrangement of elements based on their atomic number. The periodic table remains an important tool for chemists and scientists alike to this day. His predictions were later proven correct when these elements were discovered. However, there were some cases where the properties didn't match up perfectly, so Mendeleev left gaps in the table for undiscovered elements. To reflect this, he arranged the elements in rows and columns, with similar elements placed above and below each other. Mendeleev initially ordered the elements by atomic mass, but he soon noticed that every eight elements or so shared certain properties. It was first developed in 1869 by a Russian chemist named Dmitri Mendeleev, who built on the work of other scientists like John Newlands. The periodic table is a fascinating creation that we still use today.

:max_bytes(150000):strip_icc()/PeriodicTable_AtomSizes-56a131193df78cf772684720.png)
So, if you want to know more about periodic trends, keep reading! What are periodic trends? We'll even cover density and electrical conductivity. By the end of this article, you'll be able to describe and explain trends in things like electron configuration, atomic radius, and first ionisation energy. We'll also take a look at periodic trends as you move along a period in the periodic table and as you move down a group. In this article, we'll explore periodic trends in inorganic chemistry and explain what periodicity is. This is because of something called periodicity, which means that certain trends in element properties repeat in regular intervals as you move along the table. It can actually help you predict the properties of an element just by knowing where it is located on the table. The periodic table is more than just a chart of elements.


 0 kommentar(er)
0 kommentar(er)
